With gene-editing techniques such as CRISPR-Cas9, offending genes could
one day be snipped out of hematopoietic stem cells, then be returned to their
owners to generate new lines of disease-free blood cells
New research has nudged scientists closer to one of regenerative medicine’s holy grails: the ability to create customised human stem cells capable of forming blood that would be safe for patients.
Advances reported in the journal Nature could not only give scientists a window on what goes wrong in such blood cancers as leukaemia, lymphoma and myeloma, but they could also improve the treatment of those cancers, which affect some 1.2 million Americans.
The stem cells that give rise to our blood are a mysterious wellspring of life. In principle, just one of these primitive cells can create much of a human being’s immune system, not to mention the complex slurry of cells that courses through a person’s arteries, veins and organs.
While the use of blood-making stem cells in medicine has been common since the 1950s, it remains pretty crude. After patients with blood cancers have undergone powerful radiation and chemotherapy treatments to kill their cancer cells, they often need a bone-marrow transplant to rebuild their white blood cells, which are destroyed by that treatment.
The blood-making stem cells that reside in a donor’s bone marrow — and in umbilical cord blood that is sometimes harvested after a baby’s birth — are called “hematopoietic,” and they can be life-saving. But even these stem cells can bear the distinctive immune system signatures of the person from whom they were harvested. As a result, they can provoke an attack if the transplant recipient’s body registers the cells as foreign.
This response, called graft-versus-host disease, affects as many as 70 percent of bone-marrow transplant recipients in the months following the treatment, and 40 percent develop a chronic version of the affliction later. It can overwhelm the benefit of a stem cell transplant. And it kills many patients.
Rather than hunt for a donor who’s a perfect match for a patient in need of a transplant — a process that can be lengthy, ethically fraught and ultimately unsuccessful — doctors would like to use a patient’s own cells to engineer the hematopoietic stem cells.
The patient’s mature cells would be “reprogrammed” to their most primitive form: stem cells capable of becoming virtually any kind of human cell. Then factors in their environment would coax them to become the specific type of stem cells capable of giving rise to blood.
Once reintroduced into the patient, the cells would take up residence without prompting rejection and set up a lifelong factory of healthy new blood cells.
If the risk of deadly rejection episodes could be eliminated, physicians might also feel more confident treating blood diseases that are painful and difficult but not immediately deadly — diseases such as sickle cell disease and immunological disorders — with stem cell transplants.
The two studies published on Wednesday demonstrate that scientists may soon be capable of pulling off the sequence of operations necessary for such treatments to move ahead.
One of two research teams, led by stem cell pioneer Dr George Q. Daley of Harvard Medical School and the Dana Farber Cancer Institute in Boston, started their experiment with human “pluripotent” stem cells — primitive cells capable of becoming virtually any type of mature cell in the body. Some of them were embryonic stem cells and others were induced pluripotent stem cells, or iPS cells, which are made by converting mature cells back to a flexible state.
The scientists then programmed those pluripotent stem cells to become endothelial cells, which line the inside of certain blood vessels. Past research had established that those cells are where blood-making stem cells are born.
Here, the process needed a nudge. Using suppositions gleaned from experiments with mice, Daley said his team confected a “special sauce” of proteins that sit on a cell’s DNA and programme its function. When they incubated the endothelial cells in the sauce, they began producing hematopioetic stem cells in their earliest form.
Daley’s team then transferred the resulting blood-making stem cells into the bone marrow of mice to see if they would “take.” In two out of five mice who got the most promising cell types, they did. Not only did the stem cells establish themselves, they continued to renew themselves while giving rise to a wide range of blood cells.
A second research team, led by researchers from Weill Cornell Medicine’s Ansary Stem Cell Institute in New York, achieved a similar result using stem cells from the blood-vessel lining of adult mice. After programming those cells to revert to a more primitive form, the scientists also incubated those stem cells in a concoction of specialised proteins.
When the team, led by Raphael Lis and Dr Shahin Rafii, transferred the resulting stem cells back into the tissue lining the blood vessels of the mice from which they came, that graft also “took.” For at least 40 weeks after the incubated stem cells were returned to their mouse owners, the stem cells continued to regenerate themselves and give rise to many blood-cell types without provoking immune reactions.
In addition to making a workhorse treatment for blood cancers safer, the new advances may afford scientists a unique window on the mechanisms by which blood diseases take hold and progress, said Lee Greenberger, chief scientific officer for the Leukemia and Lymphoma Society.
“From a research point of view you could now actually begin to model diseases,” said Greenberger. “If you were to take the cell that’s defective and make it revert to a stem cell, you could effectively reproduce the disease and watch its progression from the earliest stages.”
That, in turn, would make it easier to narrow the search for drugs that could disrupt that disease process early. And it would speed the process of discovering which genes are implicated in causing diseases. With gene-editing techniques such as CRISPR-Cas9, those offending genes could one day be snipped out of hematopoietic stem cells, then be returned to their owners to generate new lines of disease-free blood cells.
But Daley cautioned that significant hurdles remain before studies like these will transform the treatment of blood diseases.
“We do know the resulting cells function like blood stem cells, but they still are at some distance, molecularly, from native stem cells,” he said. By tinkering with the processes by which pluripotent stem cells mature into blood-producing stem cells, Daley said his team hopes to make these lab-grown cells a better match for the real things. —Los Angeles Times/TNS
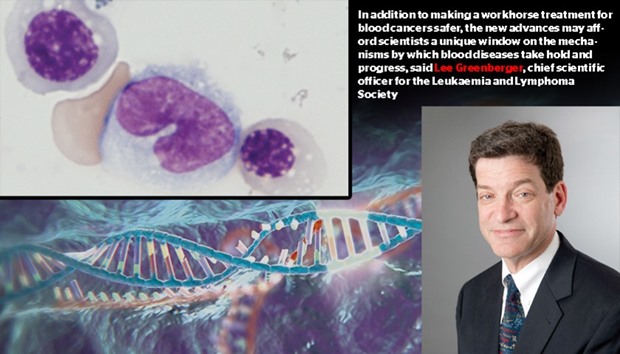
(TOP LEFT PHOTO) BREAKTHROUGH: Human blood cells grown from induced pluripotent stem cells. New techniques described in the journal Nature could make it possible for doctors to treat cancer patients with their own cells.
