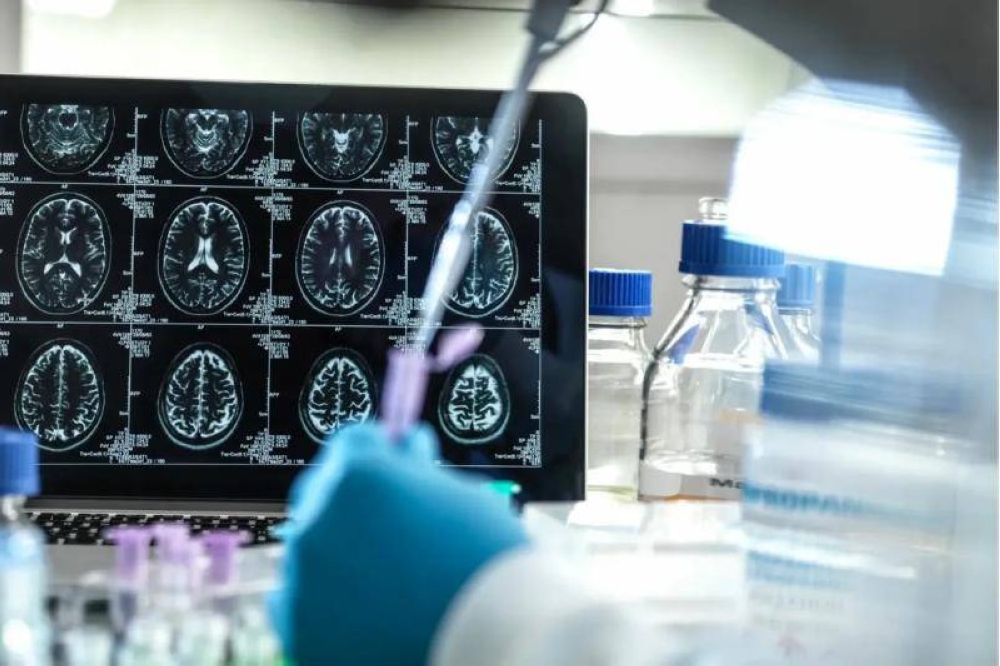
The US Food and Drug Administration (FDA) on Tuesday approved Kisunla (donanemab-azbt) injection, produced by Eli Lilly, for the treatment of Alzheimers disease.Kisunla is the third amyloid-targeting drug approved by the FDA since 2021, following two treatments Aduhelm and Leqembi.Kisunla slowed the cognitive and functional decline in patients with mild cognitive impairment by 35 percent over 18 months.The drug removes beta-amyloid that accumulates in the brains of patients with Alzheimer's disease.The estimated cost for a six-month course of the therapy is $12,522. A full year of treatment is protected to cost $32,000, according to Eli Lilly.